Reify Health
Founded Year
2012Stage
Series D | AliveTotal Raised
$483.64MValuation
$0000Last Raised
$220M | 3 yrs agoMosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
-5 points in the past 30 days
About Reify Health
Reify Health specializes in optimizing clinical trial operations within the healthcare sector. The company offers cloud-based software solutions designed to accelerate patient enrollment in clinical trials, aiming to streamline the process of bringing new therapies to patients. Reify Health primarily serves the biopharmaceutical industry, research clinics, and healthcare organizations involved in clinical research. Reify Health was formerly known as ZeroSum Health. It was founded in 2012 and is based in Boston, Massachusetts.
Loading...
ESPs containing Reify Health
The ESP matrix leverages data and analyst insight to identify and rank leading companies in a given technology landscape.
The trial recruitment optimization tools market streamlines the process of finding and enrolling participants in clinical trials. These platforms offer tech-enabled solutions to quickly match potential participants with suitable clinical trials, from marketplaces to proprietary databases. By automating the identification, pre-screening, and matching of candidates to specific trials, these systems …
Reify Health named as Leader among 15 other companies, including Microsoft, Amazon, and Salesforce.
Loading...
Research containing Reify Health
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Reify Health in 4 CB Insights research briefs, most recently on May 23, 2025.
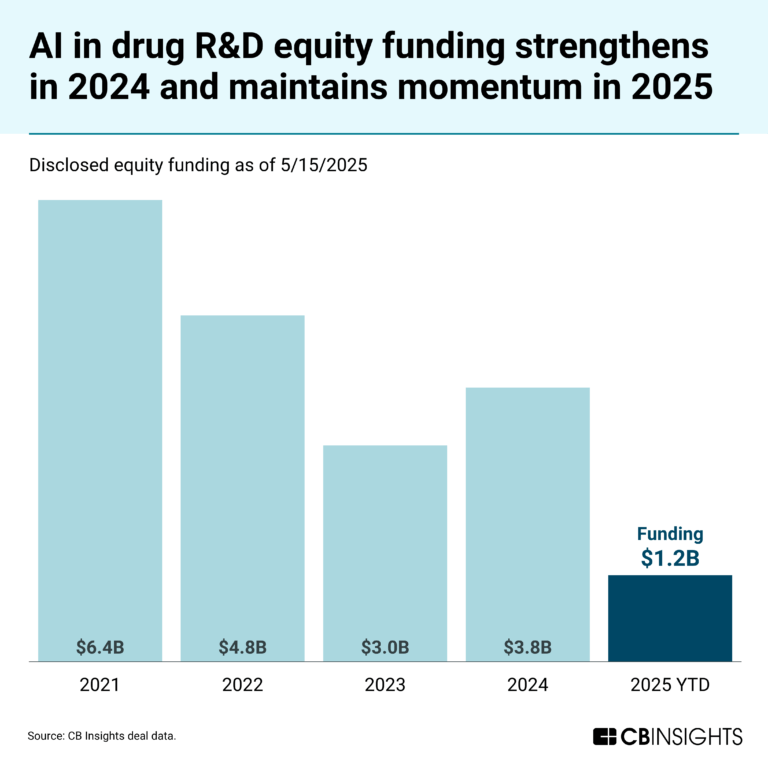
May 23, 2025
The AI in drug R&D market map
Aug 21, 2024
The clinical trials tech market map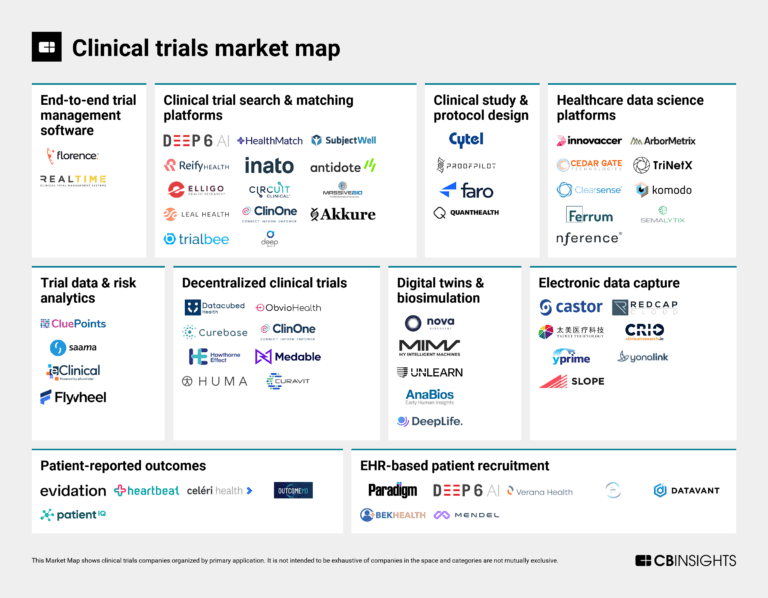
Aug 1, 2023
The clinical trials market mapExpert Collections containing Reify Health
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Reify Health is included in 3 Expert Collections, including Unicorns- Billion Dollar Startups.
Unicorns- Billion Dollar Startups
1,276 items
Digital Health
11,408 items
The digital health collection includes vendors developing software, platforms, sensor & robotic hardware, health data infrastructure, and tech-enabled services in healthcare. The list excludes pureplay pharma/biopharma, sequencing instruments, gene editing, and assistive tech.
Digital Health 50
150 items
The winners of the third annual CB Insights Digital Health 150.
Reify Health Patents
Reify Health has filed 2 patents.

Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
1/6/2010 | 4/7/2015 | Fractals, Dynamical systems, Chemical processes, Systems theory, Robotics | Grant |
Application Date | 1/6/2010 |
|---|---|
Grant Date | 4/7/2015 |
Title | |
Related Topics | Fractals, Dynamical systems, Chemical processes, Systems theory, Robotics |
Status | Grant |
Latest Reify Health News
Oct 21, 2024
News Provided By Share This Article Virtual Clinical Trials Global Market Report 2024 – Market Size, Trends, And Global Forecast 2024-2033 The Business Research Company's Virtual Clinical Trials Global Market Report 2024 – Market Size, Trends, And Global Forecast 2024-2033 The virtual clinical trials market size is expected to see rapid growth in the next few years. It will grow to $17.43 billion in 2028 at a compound annual growth rate (CAGR) of 10.2%. ” — The Business Research Company LONDON, GREATER LONDON, UNITED KINGDOM, October 21, 2024 / EINPresswire.com / -- The virtual clinical trials market has experienced robust growth in recent years, expanding from $10.81 billion in 2023 to $11.83 billion in 2024 at a compound annual growth rate (CAGR) of 9.4%. The growth in the historic period can be attributed to increased costs of clinical trials, growing need for patient-centric trials, increased demand for remote monitoring, growing adoption of digital technologies in clinical trials, increased need for efficient and cost-effective clinical trial. The virtual clinical trials market is projected to continue its strong growth, reaching $17.43 billion in 2028 at a compound annual growth rate (CAGR) of 10.2%. The growth in the forecast period can be attributed to growing adoption of decentralized clinical trials, increasing use of wearables and other remote monitoring devices, expansion of telehealth services, regulatory support for virtual trial methodologies, cost and time efficiency in trial conduct. Explore Comprehensive Insights Into The Global Virtual Clinical Trials Market With A Detailed Sample Report: Growth Driver Of The Virtual Clinical Trials Market The increasing number of clinical trials is expected to propel the growth of the virtual clinical trials market going forward. Clinical trials are research studies conducted on human participants to evaluate the safety, efficacy, and potential benefits of new medical treatments, interventions, or drugs. The rise in clinical trials has paved the way for the growth and development of virtual clinical trials, offering a more efficient, cost-effective, and patient-friendly approach to conducting clinical research. Explore The Report Store To Make A Direct Purchase Of The Report: Which Market Players Are Driving The Virtual Clinical Trials Market Growth? Key players in the market include ICON plc, Parexel International Pvt. Ltd., IQVIA Holdings Inc., Covance Research Products Inc., Pharmaceutical Research Associates Inc., Medidata Solution Inc., Oracle Corporation, Clario Tech Ltd., Medable Inc., Science 37 Holdings Inc., THREAD Research, Clinical Ink Inc., Veeva Systems Inc., Clinerion Ltd., CRF Health Group Limited, Royal Castor Products Limited, Crio Inc., Deep Lens Inc., Florence Healthcare Inc., goBalto Inc., Medrio Inc., Mint Medical Ltd., Mytrus Inc., OpenClinica LLC, PatientWing, RealTime-CTMS LLC, Reify Health, Sanguine Biosciences Inc., TriNetX Inc., VirTrial LLC What Are The Emerging Trends Shaping The Virtual Clinical Trials Market Size? Major companies operating in the virtual clinical trials market are increasing their focus on partnerships to boost virtual clinical trials. Strategic partnerships refer to a process in which companies leverage each other's strengths and resources to achieve mutual benefits and success. How Is The Global Virtual Clinical Trials Market Segmented? 1) By Design: Observational Trials, Interventional Trials, Expanded Access Trials 2) By Phases: Phase I, Phase II, Phase III, Phase IV 3) By Indication: CNS, Autoimmune/Inflammation, Cardiovascular Disease, Metabolic/Endocrinology, Infectious Disease, Oncology, Genitourinary, Ophthalmology, Other Indications Geographical Insights: North America Leading The Virtual Clinical Trials Market North America was the largest region in the market in 2023. Asia-Pacific is expected to be the fastest-growing region in the report during the forecast period. The regions covered in the report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The virtual clinical trials refers to remote or decentralized clinical trials or studies that incorporate digital health technologies and enable remote participation outside the traditional brick-and-mortar clinical trial site using tablets, smartphone apps, or wearable sensors. Virtual clinical trials allow significant digital changes in clinical research methodology, resulting in a more patient-centric ecosystem. Virtual Clinical Trials Global Market Report 2024 from The Business Research Company covers the following information: • Market size data for the forecast period: Historical and Future • Macroeconomic factors affecting the market in the short and long run • Analysis of the macro and micro economic factors that have affected the market in the past five years • Market analysis by region: Asia-Pacific, China, Western Europe, Eastern Europe, North America, USA, South America, Middle East and Africa. • Market analysis by countries: Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA. An overview of the global virtual clinical trials market report covering trends, opportunities, strategies, and more The Virtual Clinical Trials Global Market Report 2024 by The Business Research Company is the most comprehensive report that provides insights on virtual clinical trials market size, virtual clinical trials market drivers and trends and virtual clinical trials market growth across geographies. This report helps you gain in-depth insights into opportunities and strategies. Companies can leverage the data in the report and tap into segments with the highest growth potential. Browse Through More Similar Reports By The Business Research Company: Virtual Reality In Healthcare Global Market Report 2024
Reify Health Frequently Asked Questions (FAQ)
When was Reify Health founded?
Reify Health was founded in 2012.
Where is Reify Health's headquarters?
Reify Health's headquarters is located at 33 Arch Street, Boston.
What is Reify Health's latest funding round?
Reify Health's latest funding round is Series D.
How much did Reify Health raise?
Reify Health raised a total of $483.64M.
Who are the investors of Reify Health?
Investors of Reify Health include Battery Ventures, Adams Street Partners, Coatue, Dragoneer Investment Group, Altimeter Capital and 8 more.
Who are Reify Health's competitors?
Competitors of Reify Health include Castor, ObvioHealth, Science 37, Pentavere Research Group, Curebase and 7 more.
Loading...
Compare Reify Health to Competitors

Medable is a company that offers a cloud-based platform for decentralized clinical trial technology in the healthcare and clinical research sectors. The platform facilitates electronic Clinical Outcome Assessments (eCOA), remote monitoring, and the integration of connected healthcare sensors for clinical trials. Medable was formerly known as Dermatrap. It was founded in 2012 and is based in Palo Alto, California.

Curebase is a company that provides an eClinical platform in the clinical research sector, offering tools such as electronic patient-reported outcomes (ePRO), electronic clinical outcome assessments (eCOA), electronic consent (eConsent), and electronic data capture (EDC) systems. The platform is designed for study launches, data collection, and participant engagement. It was founded in 2017 and is based in San Francisco, California.

THREAD is a company focused on clinical research and electronic clinical outcome assessments (eCOA) within the life sciences sector. It offers a proprietary decentralized research platform and a suite of supporting services designed to enable remote data capture from participants and sites during clinical studies. It was founded in 2005 and is based in Tustin, California.

Formation Bio focuses on improving drug development within the pharmaceutical industry. The company specializes in acquiring clinical-stage assets and using its proprietary technology platform to enhance the drug development process. Formation Bio serves the pharmaceutical and biotech sectors, working to connect medical research and patient access to new treatments. Formation Bio was formerly known as TrialSpark. It was founded in 2013 and is based in New York, New York.

ObvioHealth provides digital health solutions within the clinical trial industry. The company has a platform and mobile application allows for remote monitoring and participation in clinical trials, focusing on data collection and participant engagement. ObvioHealth's services are relevant to the healthcare sector, especially in trial management. It was founded in 2017 and is based in New York, New York.

Bloqcube specializes in clinical trial management software within the life science industry. Its platform aggregates data in real-time and aims to assist small and midsize life-science companies. Bloqcube's services are directed towards the life science sector, focusing on clinical trials. It was founded in 2017 and is based in Piscataway, New Jersey.
Loading...