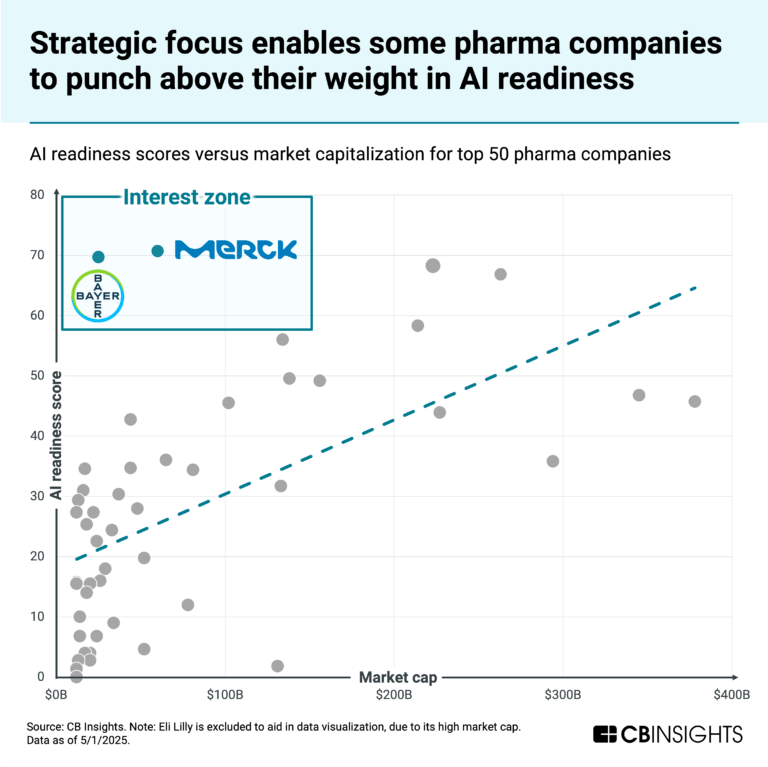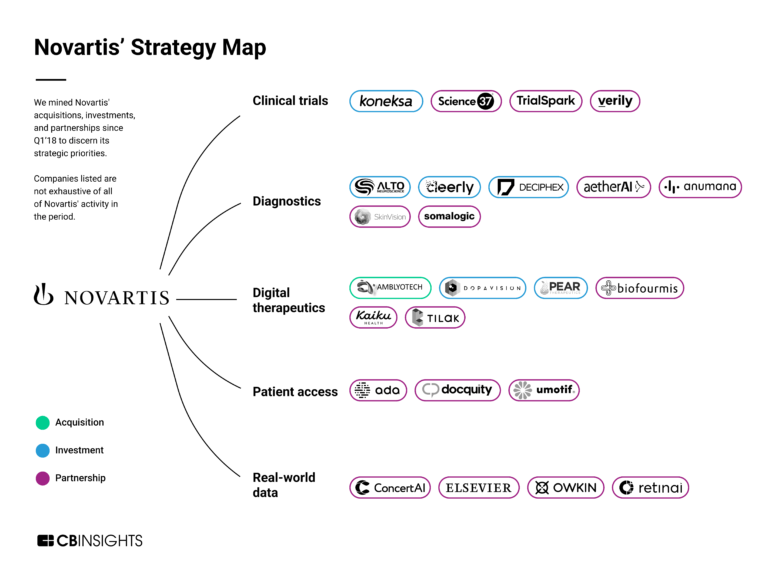
Alto Neuroscience
Founded Year
2019Stage
IPO | IPOTotal Raised
$180.17MDate of IPO
2/2/2024Market Cap
0.06BStock Price
2.35Revenue
$0000About Alto Neuroscience
Alto Neuroscience develops medicines for neuropsychiatric conditions within the pharmaceutical industry. The company creates clinical-stage drug candidates aimed at treating major depressive disorder, bipolar depression, and schizophrenia, using neurobiological research for their development. Alto Neuroscience sells to the healthcare sector, focusing on psychiatry and personalized medicine. It was founded in 2019 and is based in Los Altos, California.
Loading...
Loading...
Research containing Alto Neuroscience
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Alto Neuroscience in 2 CB Insights research briefs, most recently on Jul 3, 2025.
Expert Collections containing Alto Neuroscience
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Alto Neuroscience is included in 4 Expert Collections, including Digital Health.
Digital Health
11,408 items
The digital health collection includes vendors developing software, platforms, sensor & robotic hardware, health data infrastructure, and tech-enabled services in healthcare. The list excludes pureplay pharma/biopharma, sequencing instruments, gene editing, and assistive tech.
Precision Medicine Tech Market Map
160 items
This CB Insights Tech Market Map highlights 160 precision medicine companies that are addressing 9 distinct technology priorities that pharmaceutical companies and healthcare providers face.
Mental Health Tech
791 items
This collection includes companies applying technology to problems of emotional, psychological, and social well-being. Examples include companies working in areas such as substance abuse, eating disorders, stress reduction, depression, PTSD, and anxiety.
Artificial Intelligence
10,047 items
Alto Neuroscience Patents
Alto Neuroscience has filed 16 patents.
The 3 most popular patent topics include:
- antidepressants
- depression (psychology)
- psychiatric diagnosis

Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
5/21/2024 | 2/25/2025 | Psychiatric diagnosis, Piperazines, Antidepressants, Depression (psychology), Abnormal psychology | Grant |
Application Date | 5/21/2024 |
|---|---|
Grant Date | 2/25/2025 |
Title | |
Related Topics | Psychiatric diagnosis, Piperazines, Antidepressants, Depression (psychology), Abnormal psychology |
Status | Grant |
Latest Alto Neuroscience News
Jun 30, 2025
neuropsychiatric disorders, today highlighted The Lancet Psychiatry publication of data from the PAX-D study evaluating pramipexole in patients with treatment-resistant depression (TRD).The study was conducted by the University of Oxford and was funded by the UK government’s National Institute for Health and Care Research. Results showed pramipexole augmentation of antidepressant treatment, at a target dose of 2.5mg, demonstrated a large (Cohen’s d =0.87) reduction in symptoms relative to placebo at 12 weeks, but was associated with a high rate of adverse effects. The link to the online publication can be found. The PAX-D study results guided Alto’s acquisition of ALTO-207, a fixed-dose combination of pramipexole and the antiemetic, ondansetron. This press release features multimedia. View the full release here: “Publication in an esteemed peer-reviewed journal like The Lancet Psychiatry underscores the significance of these findings and therapeutic potential of ALTO-207 to address a critical gap in TRD,” said Amit Etkin, M.D., Ph.D., founder and chief executive officer of Alto Neuroscience. “ALTO-207 is designed to consistently achieve rapid antidepressant effect through faster titration, while mitigating the dose-limiting nausea and vomiting experienced with pramipexole alone. As we prepare to initiate our potentially pivotal, Phase 2b trial by mid-2026, we look forward to drawing on our proprietary insights on dopamine biomarkers in depression and partnering with the National Health Service network, including PAX-D sites to expand our clinical footprint and maximize the likelihood of success.” The PAX-D sites are supported by the National Institute for Health and Care Research (NIHR) Mental Health Translational Research Collaboration (MH-TRC) mission. Michael Browning, DPhil, MB.BS, MRCP, MRCPsych, Professor of Computational Psychiatry, University of Oxford, and lead study author added, “As a physician, I am encouraged by the robust and durable clinical effects seen for pramipexole in patients with TRD. While pramipexole may offer greater antidepressant effects than other available TRD treatments, the slow titration aimed at mitigating dose-limiting AEs is likely to hinder adoption. These results make it clear that optimizing tolerability to overcome current barriers may lead to a paradigm shift in treatment.” Professor Browning presented results from The Lancet Psychiatry publication during Alto’s recent investor conference call to discuss the acquisition of ALTO-207. A replay of the webcast is accessible on the Company’s website. About ALTO-207 ALTO-207 (formerly known as CTC-501) is a fixed-dose combination of pramipexole, a dopamine D3-preferring D3/D2 agonist, approved for the treatment of Parkinson’s disease with demonstrated antidepressant effect, and ondansetron, an antiemetic, selective 5-HT3 receptor antagonist. As a fixed-dose combination, ALTO-207 is designed to enable rapid titration and higher dosing by mitigating the dose-limiting adverse events typically experienced with pramipexole. ALTO-207 is being developed to address the significant unmet need for patients with TRD. Chase Therapeutics Corporation, prior to asset acquisition by Alto, completed a randomized, placebo-controlled Phase 2a clinical trial evaluating CTC-501 in 32 patients with depression. CTC-501 met primary and secondary endpoints demonstrating significantly greater improvements on MADRS compared to placebo. Patients randomized to receive CTC-501 reached a mean dose of 4.1mg per day. CTC-501 was well tolerated in the maintenance period of the study with an adverse event rate similar to placebo. About Alto Neuroscience Alto Neuroscience is a clinical-stage biopharmaceutical company with a mission to redefine psychiatry by leveraging neurobiology to develop personalized and highly effective treatment options. Alto’s Precision Psychiatry Platform™ measures brain biomarkers by analyzing EEG activity, neurocognitive assessments, wearable data, and other factors to better identify which patients are more likely to respond to Alto product candidates. Alto’s clinical-stage pipeline includes novel drug candidates in bipolar depression, major depressive disorder, treatment resistant depression (TRD), and schizophrenia, and other mental health conditions. For more information, visit or. Forward-Looking Statements This press release may contain forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements may be identified by words such as “expects,” “plans,” “will” and variations of these words or similar expressions that are intended to identify forward-looking statements, although not all forward-looking statements contain these words. Forward-looking statements in this press release include, but are not limited to, statements regarding Alto’s expectations for the timing, progress, and results of the planned ALTO-207 study, Alto’s expectations about the potential benefits, activity, effectiveness and safety of its product candidates and Precision Psychiatry Platform (“Platform”); and Alto’s expectations with regard to the design and results of its clinical trials. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various factors, including uncertainties inherent in the initiation, progress and completion of the planned ALTO-207 study and clinical development of ALTO-207; the risk that Alto may not achieve targeted enrollment in the ALTO-207 study or that enrollment may take longer than expected; the availability and timing of results from the ALTO-207 study; and other important factors, any of which could cause Alto’s actual results to differ from those contained in the forward-looking statements, which are described in greater detail in the section titled “Risk Factors” in Alto’s Annual Report on Form 10-K for the fiscal year ended December 31, 2024 filed with the Securities and Exchange Commission (“SEC”) as well as in other filings Alto may make with the SEC in the future. Any forward-looking statements contained in this press release speak only as of the date hereof, and Alto expressly disclaims any obligation to update any forward-looking statements contained herein, whether because of any new information, future events, changed circumstances or otherwise, except as required by law. Availability of Information on Alto’s Website Alto routinely uses its investor relations to post presentations to investors and other important information, including information that may be material. Accordingly, Alto encourages investors and others interested in Alto to review the information it makes public on its investor relations website. View source version on businesswire.com:
Alto Neuroscience Frequently Asked Questions (FAQ)
When was Alto Neuroscience founded?
Alto Neuroscience was founded in 2019.
Where is Alto Neuroscience's headquarters?
Alto Neuroscience's headquarters is located at 369 South San Antonio Road, Los Altos.
What is Alto Neuroscience's latest funding round?
Alto Neuroscience's latest funding round is IPO.
How much did Alto Neuroscience raise?
Alto Neuroscience raised a total of $180.17M.
Who are the investors of Alto Neuroscience?
Investors of Alto Neuroscience include What If Ventures, Windham Venture Partners, Alpha Wave Global, Lightswitch Capital, InVivium Capital and 20 more.
Who are Alto Neuroscience's competitors?
Competitors of Alto Neuroscience include Monument Therapeutics and 5 more.
Loading...
Compare Alto Neuroscience to Competitors

Quadrant Biosciences is a life sciences company that specializes in the early detection of neurological disorders and other health conditions. The company offers diagnostic solutions using saliva-based molecular diagnostics to identify markers for conditions such as autism spectrum disorder, Parkinson's disease, and mild traumatic brain injury (mTBI), as well as RNA analysis for COVID-19 detection. Quadrant Biosciences was formerly known as Motion Intelligence. It was founded in 2012 and is based in Syracuse, New York.

BrainCheck focuses on cognitive health and operates in the healthcare technology sector. The company offers a digital platform that provides cognitive assessments, clinical decision support, and cognitive care planning. Its services are designed to help healthcare providers identify and manage dementia and cognitive impairment in their patients. It was founded in 2015 and is based in Austin, Texas.
Biotrial is a Contract Research Organization (CRO) that specializes in drug development within the pharmaceutical and biotech industries. The company offers a wide range of services including preclinical pharmacology, clinical development, bioanalysis, biometrics, and medical imaging. Biotrial primarily serves the pharmaceutical and biotech industries. It was founded in 1989 and is based in Rennes, France.
Myelin-H focuses on the remote monitoring and management of brain diseases within the healthcare technology sector. The company offers a platform that facilitates the tracking and analysis of patient data for brain conditions. This technology serves as a tool for healthcare professionals to manage disease progression remotely. It was founded in 2021 and is based in London, United Kingdom.
Biomind Labs is a biotech research and development company operating in the pharmaceutical industry. The company focuses on developing novel pharmaceutical drugs and innovative nanotech delivery systems for treating various psychiatric and neurological conditions. These products are designed to transform biomedical sciences knowledge into fast-acting and controlled-release drugs that target specific therapeutic indications. It is based in Toronto, Ontario.
Mindstate Design Labs provides a preclinical-stage biotechnology solution. It focused on developing psychedelic-inspired therapeutics for mental health. The company's main offerings include a platform for target discovery and phenotypic screening of altered conscious states, a pipeline of psychedelic primer compounds, and non-psychedelic probe compounds designed to modulate these states. It primarily serves the mental health therapeutics sector. Mindstate Design Labs was formerly known as Kykeon Biotechnologies. It was founded in 2021 and is based in South San Francisco, California.
Loading...